Our Campus
Manufacturing Facility
Expanding Bio Facilities with Global Standards & Certificates
CDMO facilities with state-of-the-art technology and equipment
Prestige Biologics is located in the center of the Osong Life Science Bio-cluster in Korea and is built with state-of-the-art production facilities.
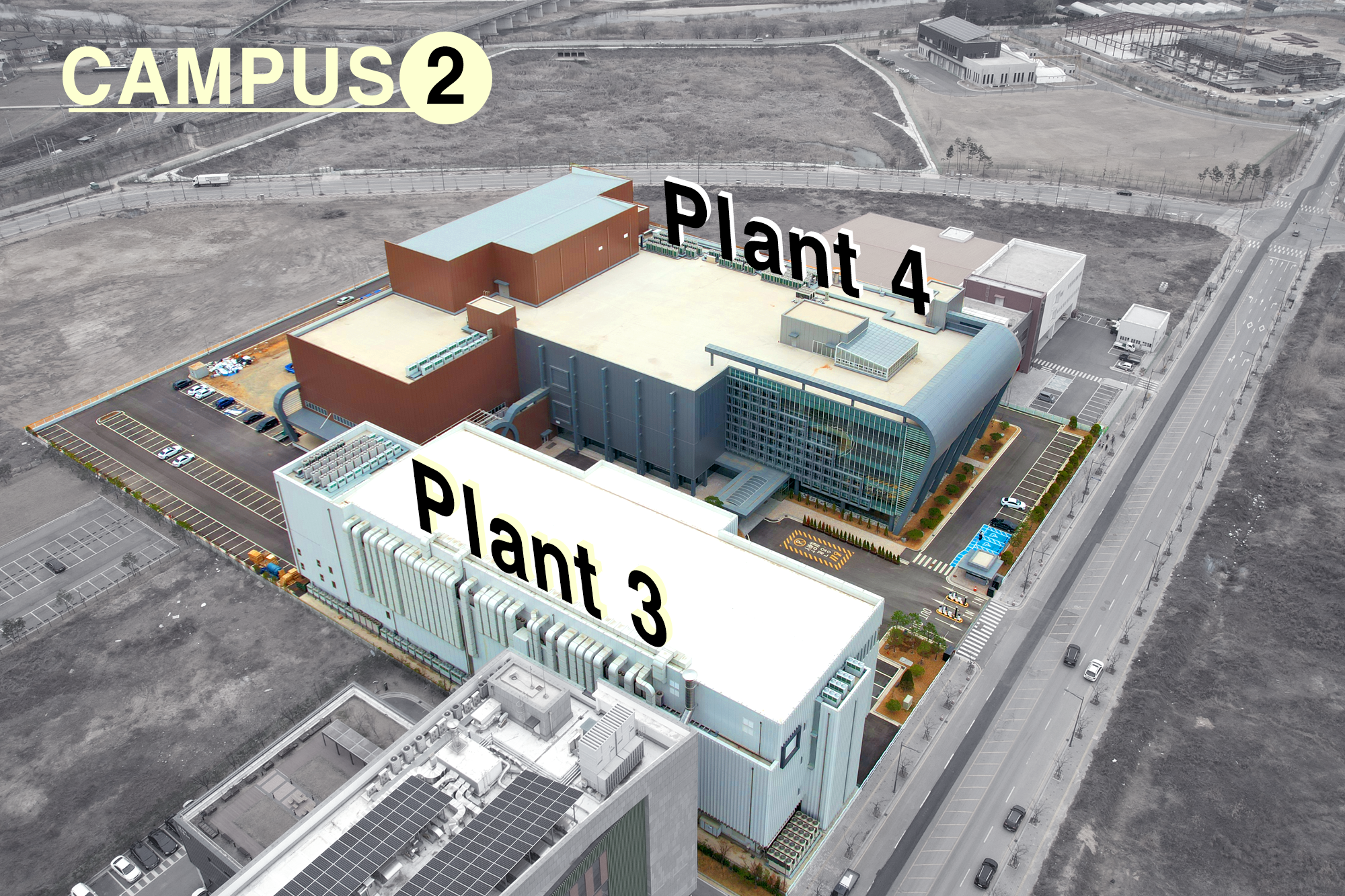
A total of four production plants (P1, P2, P3, P4) within the two campuses complete a large-scale production cluster with a total floor area of 54,000 square meters.
In addition, Prestige Biologics has secured the certification of Korea MFDS GMP and Europe EMA GMP, and have acquired ISO 9001:2015 management standards. In particular, WHO’s GDP certification was acquired through SGS, and certification for the world’s top-ranked pharmaceutical storage facilities and distribution was obtained. The procedure for cGMP certification by the US FDA is also proceeding as planned for supporting clients.
Prestige Biologics is continuously responding to and preparing for the needs of various global organizations and companies that can guarantee the quality of pharmaceuticals provided to customers.


Campus
Osong Bio-manufacturing Plants
Securing total production capacity of 154,000 L

P1 Pioneer
6,400 L
2,000 L X 3 ea
200 L X 2 ea
Production of antibodies, recombinant proteins, animal-derived cell-based medicines, etc.
Manufacturing facilities specialized in development and production supporting initial, small and critical projects

P2 Frontier
28,000L
2,000 L X 14 ea
Production of antibodies, recombinant proteins, animal-derived cell-based medicines, etc.
Manufacturing facilities that respond most flexibly to various customer needs and provide production from development

P3 Voyager
88,000L
2,000 L X 44 ea
Production of antibodies, recombinant proteins, animal-derived cell-based medicines, etc. Flexible for application to new platforms
Manufacturing facilities that provide flexible, rapid, and breakthrough production to meet market demands

P4 Adventure
32,000L
2,000 L X 16 ea
Production of antibodies, recombinant proteins, animal-derived cell-based medicines, etc.
Customizing manufacturing facility that responds to customer needs through mass production
Prestige Biologics 1st Campus
Plant 1 + Plant 2

March 2023
Prestige Biologics 2nd Campus
Plant 3 + Plant 4
March 2023
State-of-the-art equipment and facility

Equipment of global standard
Qaulity control and assurance
Prestige Biologics is establishing collaborative relationships with suppliers that provide global quality. By implementing world-class biopharmaceutical production facilities, we efficiently manage the quality of biopharmaceuticals and control risks such as cross-contamination.

Applying engineering technology at a global level.
Innovative Engineering Technology for Effectiveness
Prestige Biologics is continuously striving to maximize the efficiency of world-class equipment and enhance the productivity of biopharmaceutical manufacturing through ongoing engineering and technological advancements. We are continuously improving the capabilities of bioprocessing by incorporating innovative technology into actual production environments.
From clinical development to commercial production
Prestige Biologics provides various services such as analysis service, clinical development, clinical sample production, clinical secondary packaging (double-blind packaging), process development, and commercial production. We respond flexibly to the needs of customers of various needs, such as CDO, CPO, CMO, and CEO. We are also always open to partnerships that develop potential.




