Budget ESTIMATION Program
4 Week Budget Estimation Program
4 Week Budget
Estimation Program
Within a timeframe of 4 weeks, we propose providing estimates for budget planning in both clinical and commercial production, even for your initial stage projects.
We offer the necessary technical framework and calculate optimized production costs for you. We aim to deliver foundational estimates for reviewing essential production technologies and establishing budgets, crucial for entering the subsequent phases of clinical and commercial production.
Prestige Biologics offers a quotation service for projects at various stages, from early to late phases. For clinical and commercial production, it takes approximately 4 weeks from the initial request to provide the final proposal. Customers can not only establish phase-specific budgets for drug development but also discuss technical frameworks for competitive commercial production simultaneously.
Budget planning and technical reviews covering the entire project lifecycle are best conducted as early as possible. For instance, strategic considerations about whether clinical production will be conducted at a small-scale batch or at the scale of commercial production are made in the early stages of the project. This early planning helps manage potential unnecessary economic expenditures. Additionally, considerations for the cost of technology transfer and early estimation of key management costs for long-term project operation should also be addressed sooner rather than later.
To address these concerns in the field of biopharmaceutical development, Prestige Biologics offers a program that provides estimated quotations for key projects within 4 weeks, encapsulating the worries and considerations prevalent in the bio-pharmaceutical development landscape.
Overview of Budget Estimation Program
Process of 4 Week Budget Estimation Program
Prestige Biologics operates the following proposal presentation program:
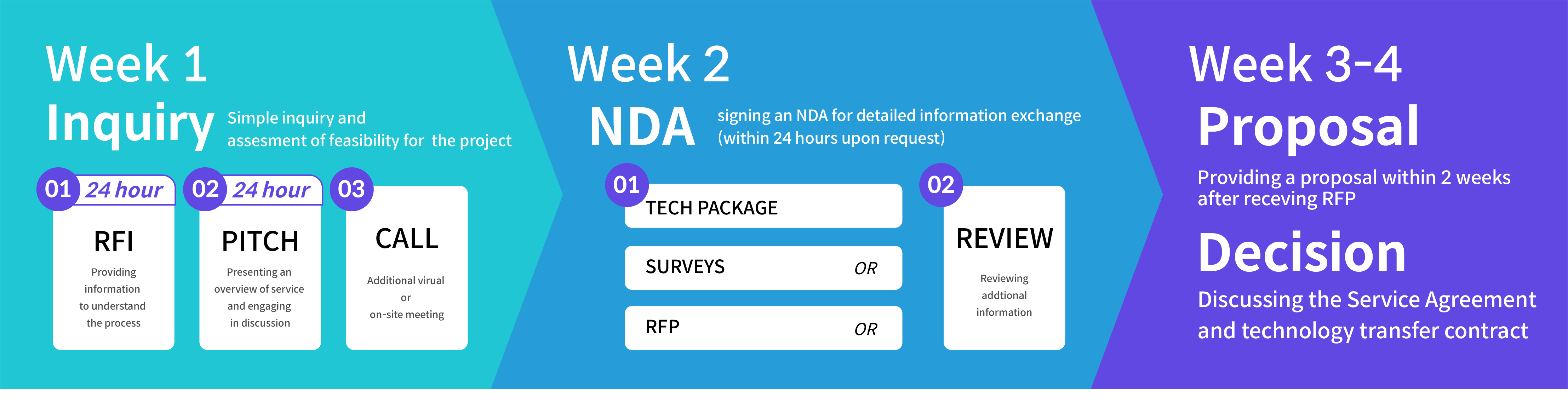
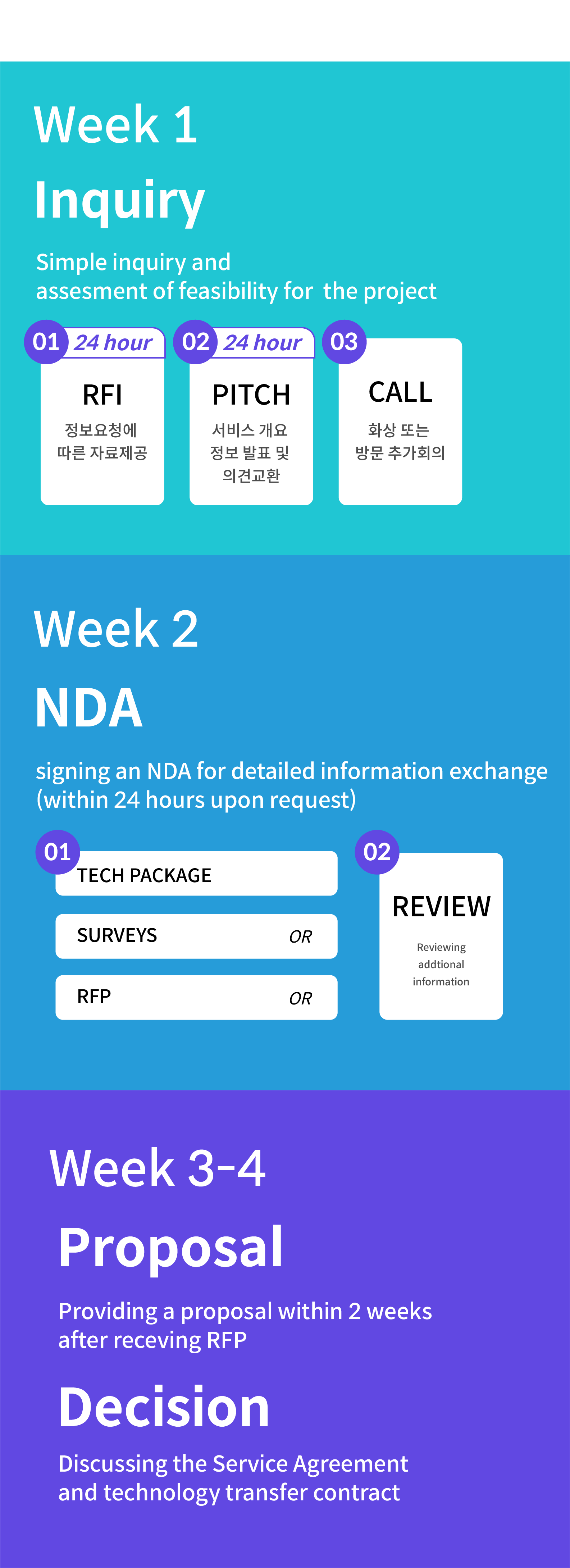
- Inquiry and Technical Verification: Initially, an assessment of the technical feasibility of the program is conducted based on customer inquiries (phone calls, emails, or visits). A swift evaluation is made regarding the feasibility of implementation based on our production facilities and technology, and a prompt response is provided.
- Information Provision and Preliminary Meetings: Introductory information about our services, such as company brochures and technical guides, is provided. Detailed introductions are conducted through visits or virtual meetings. Additional meetings are scheduled as needed. The first round of information provision and meetings is offered within the earliest possible time as requested by the customer.
- Execution of Confidential Agreements (e.g., NDA): To ensure mutual confidentiality, a Non-Disclosure Agreement (NDA) is executed. The NDA document, which serves as our basic contract, is provided within 24 hours. In cases where the customer provides their NDA form, it is signed and returned within 24 hours.
- Information Exchange and Review: Following the NDA execution, a period of approximately 3 to 10 days is dedicated to the exchange of technical information. After a consistent information exchange, we generally receive a Request for Proposal (RFP) or Technical Package from the customer.
- Proposal Submission: In accordance with the customer’s RFP, detailed technical specifications and cost estimates are provided. A proposal is submitted within approximately 2 weeks.
Process of 4 Week Budget Estimation Program
We support the successful journey of your projects.
Please register for the 4Week Budget Estimation Program.
To provide technical and quotation information for clinical and commercial production, as well as key services, please provide the following information. We will offer estimates and technical details to assist in budget planning for your valuable projects. Additionally, you can inquire about our related services or collaboration through the provided form below.
CDEMO service that contains the DNA of innovation in CMO and CDMO
The rapidly evolving pharmaceutical environment poses challenges in accurately forecasting demand. Prestige Biologics’ CDEMO services leverage the proprietary ALITA Smart BioFactory™, an adaptive engineering solution, to provide a tailored manufacturing suite for customer products.
From technology development and production at the clinical stage to commercial production supporting high competitiveness, we offer suitable technology and production suites according to your needs.
Open-door Policy for customer
Prestige Biologics has adopted an open information sharing policy to foster close collaboration for providing customer-centric services. Through our open-door policy, we actively engage in resolving key issues arising during the development and production processes, playing a collaborative role with our partners to successfully achieve shared objectives.
We believe in fulfilling our mission of contributing to technological growth and impacting patients’ lives together through the customer service goal of ‘Growth through Openness.’ We are committed to embracing the journey of growth hand in hand with our partners and contributing to the mission of improving patients’ lives.

From clinical development to commercial production
Prestige Biologics provides various services such as analysis service, clinical development, clinical sample production, clinical secondary packaging (double-blind packaging), process development, and commercial production. We respond flexibly to the needs of customers of various needs, such as CDO, CPO, CMO, and CEO. We are also always open to partnerships that develop potential.




